
by GCP Central | Mar 9, 2017 | GCP Central News
We at GCP Central are always looking for new ways to doWMO/GCP training in an even more efficient way. For students, this by only teaching them what is relevant at that moment. For research agencies and managers, by providing reports and insight into training...

by GCP Central | Mar 9, 2017 | GCP Central News
Last week, on March 1, 2017, the amended Medical Research Involving Human Subjects Act (WMO) went into effect. The Dutch Association of Medical Ethical Testing Commissions (NVMETC) and the Central Commission of Human-Related Research (CCMO) have written a testing...

by GCP Central | Nov 2, 2016 | GCP Central News
Modified from original article on www.CCMO.nl On Tuesday, October 25, 2016, the Dutch Senate approved a bill to amend the Medical Research Involving Human Subjects Act (WMO). It is expected that in practice, this amendment will allow more room to develop new treatment...

by GCP Central | Oct 13, 2016 | GCP Central News
Source: Dutch Senate website There is currently a bill in the Dutch Senate to amend the Medical Research Involving Human Subjects Act (WMO). The aim of this amendment is to expand the possibilities for conducting (non-therapeutic) medical-scientific research with...

by GCP Central | Oct 13, 2016 | GCP Central News
It’s happened: on November 17, 2016 the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) adopted the revisions of the Good Clinical Practice directive (ICH-GCP E6 R2). Does that mean that the...

by GCP Central | Jul 21, 2016 | GCP Central News
The ICH Guideline “Good Clinical Practice” (the E6 directive) is in revision. This revision will impact the ICH-GCP requirements worldwide. This is a special moment because this is the first change since the GCP directive 20 years ago was published....

by GCP Central | Jul 16, 2016 | GCP Central News
At the end of May we delivered the first facts about the myGCP learning environment. How is the myGCP learning environment used by students? And by whom? We found out that nearly half of our students (44%) the age of 25 – 44 years, 70% of the myGCP users are women and...

by GCP Central | Jun 30, 2016 | GCP Central News, GCP Central Products
After obtaining the WMO/GCP certificate (EMWO) you need to do refresher training every four years. The so-called re-registration or hercertificeringscursus. This is so you are aware of new developments in the field of quality assurance and regulatory, and thereby...

by GCP Central | May 26, 2016 | GCP Central News
Astrid Schut, Managing Director of WCN In the Holland Heart House, right in the center of Utrecht, you’ll find the Dutch Network for Cardiovascular Research (WCN). This is a networking organization of non-academic cardiology partnerships in 60 locations. WCN...

by GCP Central | May 20, 2016 | GCP Central News
One of the great advantages of the online myGCPtraining is that we can carry out analysis on various levels on data that we receive from our students. A simple example of data that we store is the progress of a student in the training. We also receive information...

by GCP Central | Sep 9, 2015 | GCP Central News
Blog by Ruben Timmerman, Springest E-learning is popular. Recent research by Nidap and Springest shows that nearly 75 percent of employees like to follow part of their training or education via e-learning. Nevertheless, almost everyone still prefers to follow at least...

by GCP Central | Sep 8, 2015 | GCP Central News
Learn about WMO / GCP and earn points for PE-online … The online WMO / GCP training is accredited for medical specialists, general practitioners and nurses. You do not only learn about the set-up and execution of clinical research, but you also receive your...

by GCP Central | May 6, 2015 | GCP Central News
Our online and blended training meet the minimum requirements for ICH/GCP training courses for researchers and support staff. These requirements are drawn up by TransCelerate, an international cooperation of pharmaceutical companies. From now on their logo is on our...

by GCP Central | Jan 21, 2015 | GCP Central News
With the learning environment we want to not only offer a place where you can follow GCP trainings, we want to provide an interactive platform for anyone who is involved in clinical research. It should be a place where we can exchange knowledge and experiences, can...

by GCP Central | Dec 13, 2014 | GCP Central News
Last month we showed plans for the learning environment we are currently designing with BeSpeak. Meanwhile, the community feature has been worked on further. Often doctor/researchers say that they miss a place where they can ask experts or experts about a subject...

by GCP Central | Dec 5, 2014 | GCP Central News
PRAcarries out research into the safety and possibly the effect of medicines. Mainly with healthy volunteers and sometimes with patients. GCP Central was allowed to visit Zuidlaren, the specialized clinic where this type of research is carried out. We saw how Good...

by GCP Central | Dec 3, 2014 | GCP Central News
The Faculty of social and behavioural sciences, health-medical section and Neuropsychology (GMN) of Leiden University is working hard to set a quality standard for their research. GCP Central was invited to give a WMO / GCP training. Behavioral sciences research has...

by GCP Central | Nov 7, 2014 | GCP Central News
In mid-2016, the new European regulations will come into effect once the European database (the ‘portal’) is ready. From that moment on, the submission process for drug research will be aligned for all EU Member States. The idea is that the new regulation...

by GCP Central | Nov 6, 2014 | GCP Central News
In mid-2016, the new European regulations will come into effect once the European database (the ‘portal’) is ready. From that moment on, the submission process for drug research will be aligned for all EU Member States. The idea is that the new regulation...
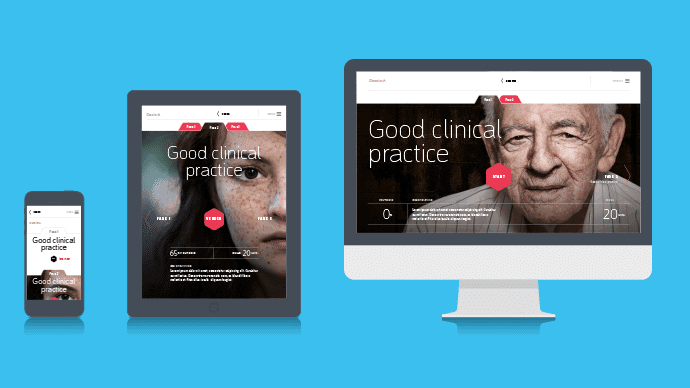
by GCP Central | Nov 3, 2014 | GCP Central News
Our learning environment is getting more and more shape! Together with our partner BeSpeak we will design a GCP platform where you will find GCP training, cases, news and GCP experiences of others. From February 2015 you can put together your own GCP training through...

by GCP Central | Sep 26, 2014 | GCP Central News
Founder of GCP Central, Marieke Meulemans, talks about the origin of the company. “In 2003 I started my working life at Kendle on the Department quality control (QC)I checked the quality of the data from the drug trials, the files were completely checked, etc....
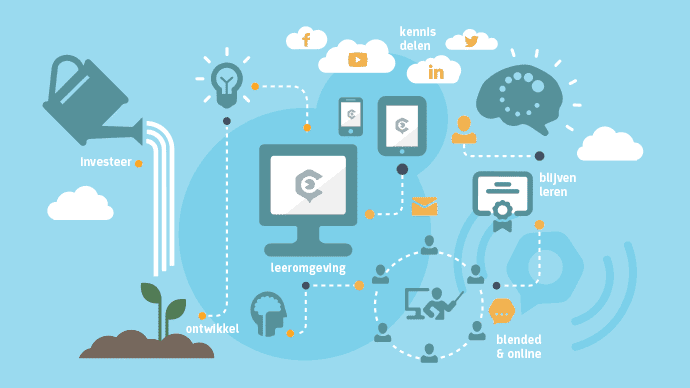
by GCP Central | Aug 25, 2014 | GCP Central News
In February 2015, we launched a unique learning environment. A platform with online training, practical cases of researchers, tools and a network of professionals. Interested parties can co-build with us by investing. For that you will not only get a return, but also...
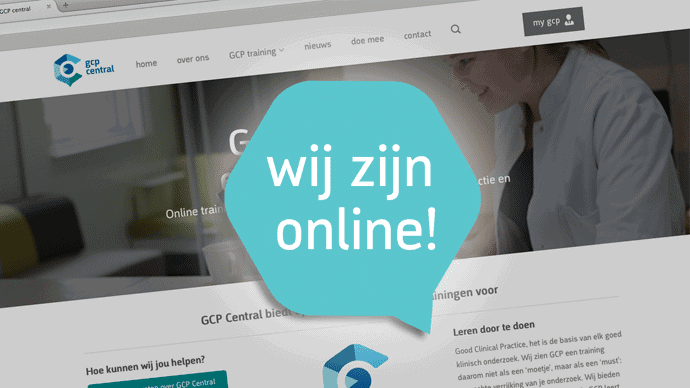
by GCP Central | Aug 22, 2014 | GCP Central News
A lot has happened since the creation of GCP Central in 2012. We now have two locations, our team is reinforced with four people and we entered into a partnership with BeSpeakfor the development of our new learning environment. In February 2015 we will launch this new...



























