
by GCP Central | Oct 11, 2018 | GCP Central News
Since the launch of GCP Central back in 2012, we have worked tirelessly towards making GCP a core part of everyday practice for medical, pharmacy and clinical professionals. After 4 years of perfecting our training methodologies and experiencing continuous business...
by GCP Central | Oct 1, 2018 | GCP Central News
Training day WMO and GCP for trial medication Pharmacists & Assistants International Good Clinical Practice Guideline (ICH-GCP), EU Directives, Medical Research involving humans Act (WMO) 7.5 hours Classroom per Day) Dutch / English At least 10, maximum 20...

by GCP Central | Jun 13, 2018 | GCP Central News
GCP Central has from 1 June 2017 taken over the GCP training activities fromICH Training and adviceAns and Anjo Strik have been active in training research professionals since 1996, and at that time experienced the creation of ICH-GCP. The knowledge of Anjo and Ans...

by GCP Central | Jun 13, 2018 | GCP Central News, updates law & regulations
On June 14, 2017 the new ICH GCP is in effect: Addendum R2. In hospitals, pharmaceutical companies and CROs, the agreement has been made that a GCP training certificate obtained is valid for 2 to 4 years. But what if, in the meantime the legislation is updated as in...

by GCP Central | Jun 13, 2018 | GCP Central News
At GCP Central, we believe that it is better for the quality of clinical research, if we do not only actively train research professionals in GCP every 2-4 years, but also in the intervening period. That may sound like “more training”, so the title of this...

by GCP Central | Apr 6, 2018 | GCP Central News
This year we are organizing the GCP Central Spring SchoolIn the week of the 15th to the 19th May 2017, we will organize various in-depth sessions and master classes on a level by top speakers. For starters with ambition and for experienced professionals who understand...

by GCP Central | Mar 21, 2018 | GCP Central News
After a careful selection process, the DCRF has entered a partnership with GCP Central for the development and operation of an online training program on the ECTRThe ECTR, which comes into force in October 2018, is the abbreviation for the EU...

by GCP Central | Mar 9, 2017 | GCP Central News, GCP Central Products, updates law & regulations
We at GCP Central are always looking for new ways to doWMO/GCP training in an even more efficient way. For students, this by only teaching them what is relevant at that moment. For research agencies and managers, by providing reports and insight into training...

by GCP Central | Mar 9, 2017 | GCP Central News
We at GCP Central are always looking for new ways to doWMO/GCP training in an even more efficient way. For students, this by only teaching them what is relevant at that moment. For research agencies and managers, by providing reports and insight into training...

by GCP Central | Mar 9, 2017 | GCP Central News
Last week, on March 1, 2017, the amended Medical Research Involving Human Subjects Act (WMO) went into effect. The Dutch Association of Medical Ethical Testing Commissions (NVMETC) and the Central Commission of Human-Related Research (CCMO) have written a testing...

by GCP Central | Nov 2, 2016 | GCP Central News
Modified from original article on www.CCMO.nl On Tuesday, October 25, 2016, the Dutch Senate approved a bill to amend the Medical Research Involving Human Subjects Act (WMO). It is expected that in practice, this amendment will allow more room to develop new treatment...

by GCP Central | Oct 13, 2016 | Experiences
Since January 2016 The Tweesteden hospital and the St. Elisabeth hospital merged to form the Elisabeth-Tweesteden Hospital (ETZ). It is one of the 26 cooperating top clinical hospitals (STZ) in Netherlands. That means extra attention to training and research. Marika...

by GCP Central | Oct 13, 2016 | GCP Central News
Source: Dutch Senate website There is currently a bill in the Dutch Senate to amend the Medical Research Involving Human Subjects Act (WMO). The aim of this amendment is to expand the possibilities for conducting (non-therapeutic) medical-scientific research with...

by GCP Central | Oct 13, 2016 | GCP Central News
It’s happened: on November 17, 2016 the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) adopted the revisions of the Good Clinical Practice directive (ICH-GCP E6 R2). Does that mean that the...

by GCP Central | Jul 21, 2016 | GCP Central News
The ICH Guideline “Good Clinical Practice” (the E6 directive) is in revision. This revision will impact the ICH-GCP requirements worldwide. This is a special moment because this is the first change since the GCP directive 20 years ago was published....

by GCP Central | Jul 16, 2016 | GCP Central News
At the end of May we delivered the first facts about the myGCP learning environment. How is the myGCP learning environment used by students? And by whom? We found out that nearly half of our students (44%) the age of 25 – 44 years, 70% of the myGCP users are women and...

by GCP Central | Jun 30, 2016 | GCP Central News, GCP Central Products
After obtaining the WMO/GCP certificate (EMWO) you need to do refresher training every four years. The so-called re-registration or hercertificeringscursus. This is so you are aware of new developments in the field of quality assurance and regulatory, and thereby...

by GCP Central | Jun 29, 2016 | Experiences
By Debby Zweers, WCN training working group On 23 June, the first meeting for the kickoff of MyGCP and the round table discussion. In the Stenden hotel in Leeuwarden, we gathered a nice roundup of sites in the North. Kickoff meeting MyGCP After a smooth opening, the...

by GCP Central | Jun 29, 2016 | Experiences
For 20 years, The Vascular Research Network (VRN) brings clinical studies to Netherlands and mediates them between pharmacist, vascular internists and cardiologists. From september 2016 they go the myGCP learning environment use of GCP Central, with a unique link from...

by GCP Central | Jun 13, 2016 | Action, GCP Central Products
just time by yourself to learn. That is why we organized this year the GCP Central Summer school. A week with training sessions and master classes on your level. For starters with ambition and for experienced professionals who understand that standing still can be...

by GCP Central | May 26, 2016 | GCP Central News
Astrid Schut, Managing Director of WCN In the Holland Heart House, right in the center of Utrecht, you’ll find the Dutch Network for Cardiovascular Research (WCN). This is a networking organization of non-academic cardiology partnerships in 60 locations. WCN...

by GCP Central | May 20, 2016 | GCP Central News
One of the great advantages of the online myGCPtraining is that we can carry out analysis on various levels on data that we receive from our students. A simple example of data that we store is the progress of a student in the training. We also receive information...

by GCP Central | May 18, 2016 | Experiences
By dr Martijn van Eck, Jeroen Bosch Ziekenhuis Per 20 april was us all through the newsletter and the website of the WCN participation in GCP-Online offered. Why this new GCP course? The theory Seasoned research professionalsWCNhave already completed a minimum of...

by GCP Central | Sep 9, 2015 | GCP Central News
Blog by Ruben Timmerman, Springest E-learning is popular. Recent research by Nidap and Springest shows that nearly 75 percent of employees like to follow part of their training or education via e-learning. Nevertheless, almost everyone still prefers to follow at least...

by GCP Central | Sep 8, 2015 | GCP Central News
Learn about WMO / GCP and earn points for PE-online … The online WMO / GCP training is accredited for medical specialists, general practitioners and nurses. You do not only learn about the set-up and execution of clinical research, but you also receive your...

by GCP Central | May 6, 2015 | GCP Central News
Our online and blended training meet the minimum requirements for ICH/GCP training courses for researchers and support staff. These requirements are drawn up by TransCelerate, an international cooperation of pharmaceutical companies. From now on their logo is on our...

by GCP Central | Jan 21, 2015 | GCP Central News
With the learning environment we want to not only offer a place where you can follow GCP trainings, we want to provide an interactive platform for anyone who is involved in clinical research. It should be a place where we can exchange knowledge and experiences, can...

by GCP Central | Dec 13, 2014 | GCP Central News
Last month we showed plans for the learning environment we are currently designing with BeSpeak. Meanwhile, the community feature has been worked on further. Often doctor/researchers say that they miss a place where they can ask experts or experts about a subject...

by GCP Central | Dec 5, 2014 | GCP Central News
PRAcarries out research into the safety and possibly the effect of medicines. Mainly with healthy volunteers and sometimes with patients. GCP Central was allowed to visit Zuidlaren, the specialized clinic where this type of research is carried out. We saw how Good...

by GCP Central | Dec 3, 2014 | GCP Central News
The Faculty of social and behavioural sciences, health-medical section and Neuropsychology (GMN) of Leiden University is working hard to set a quality standard for their research. GCP Central was invited to give a WMO / GCP training. Behavioral sciences research has...

by GCP Central | Nov 7, 2014 | GCP Central News
In mid-2016, the new European regulations will come into effect once the European database (the ‘portal’) is ready. From that moment on, the submission process for drug research will be aligned for all EU Member States. The idea is that the new regulation...

by GCP Central | Nov 6, 2014 | GCP Central News
In mid-2016, the new European regulations will come into effect once the European database (the ‘portal’) is ready. From that moment on, the submission process for drug research will be aligned for all EU Member States. The idea is that the new regulation...
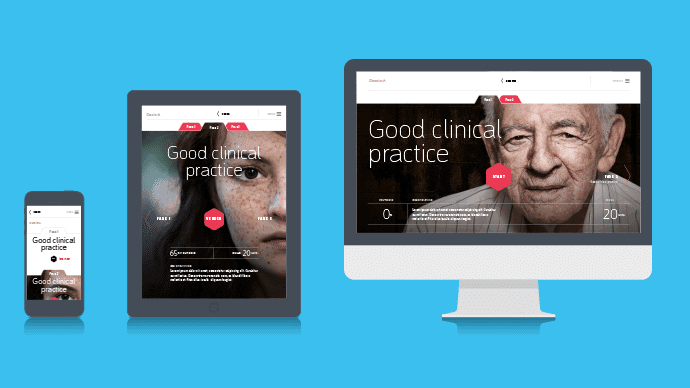
by GCP Central | Nov 3, 2014 | GCP Central News
Our learning environment is getting more and more shape! Together with our partner BeSpeak we will design a GCP platform where you will find GCP training, cases, news and GCP experiences of others. From February 2015 you can put together your own GCP training through...

by GCP Central | Nov 3, 2014 | Action
For the content of our GCP training we work together with doctors, researchers, medical directors, CRAs, auditors, research nurses and many others. Their experience, knowledge and daily dilemmas during clinical research, provide input to our cases and ensure that the...

by GCP Central | Sep 26, 2014 | GCP Central News
Founder of GCP Central, Marieke Meulemans, talks about the origin of the company. “In 2003 I started my working life at Kendle on the Department quality control (QC)I checked the quality of the data from the drug trials, the files were completely checked, etc....

by GCP Central | Aug 27, 2014 | GCP Central Products
The time of GCP training in stale training rooms with endless PowerPoints is over. GCP Central develops a unique learning concept where you learn Good Clinical Practice on the basis of relevant cases. As a student you are invited to the GCP platform: a learning...
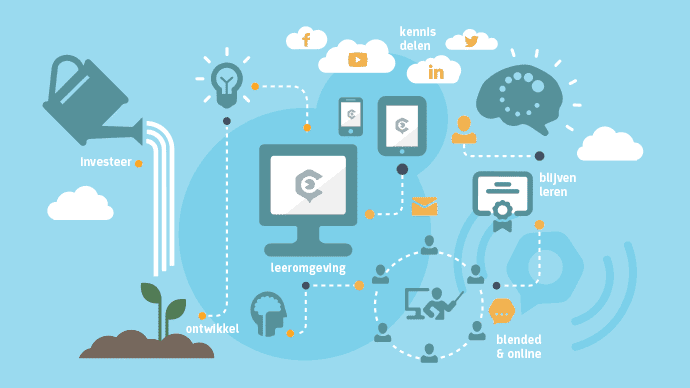
by GCP Central | Aug 25, 2014 | GCP Central News
In February 2015, we launched a unique learning environment. A platform with online training, practical cases of researchers, tools and a network of professionals. Interested parties can co-build with us by investing. For that you will not only get a return, but also...
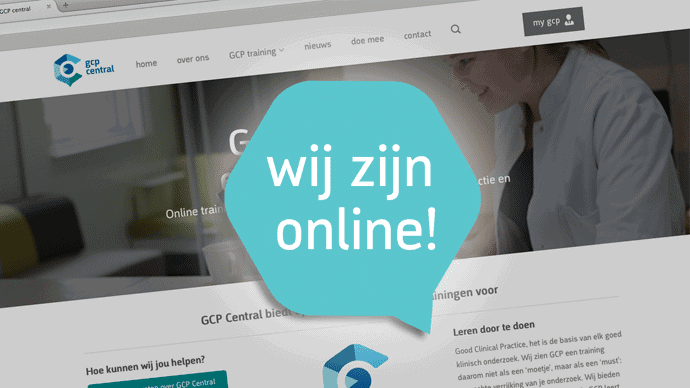
by GCP Central | Aug 22, 2014 | GCP Central News
A lot has happened since the creation of GCP Central in 2012. We now have two locations, our team is reinforced with four people and we entered into a partnership with BeSpeakfor the development of our new learning environment. In February 2015 we will launch this new...









































