gcp training courses
EXPLORE TOPICS




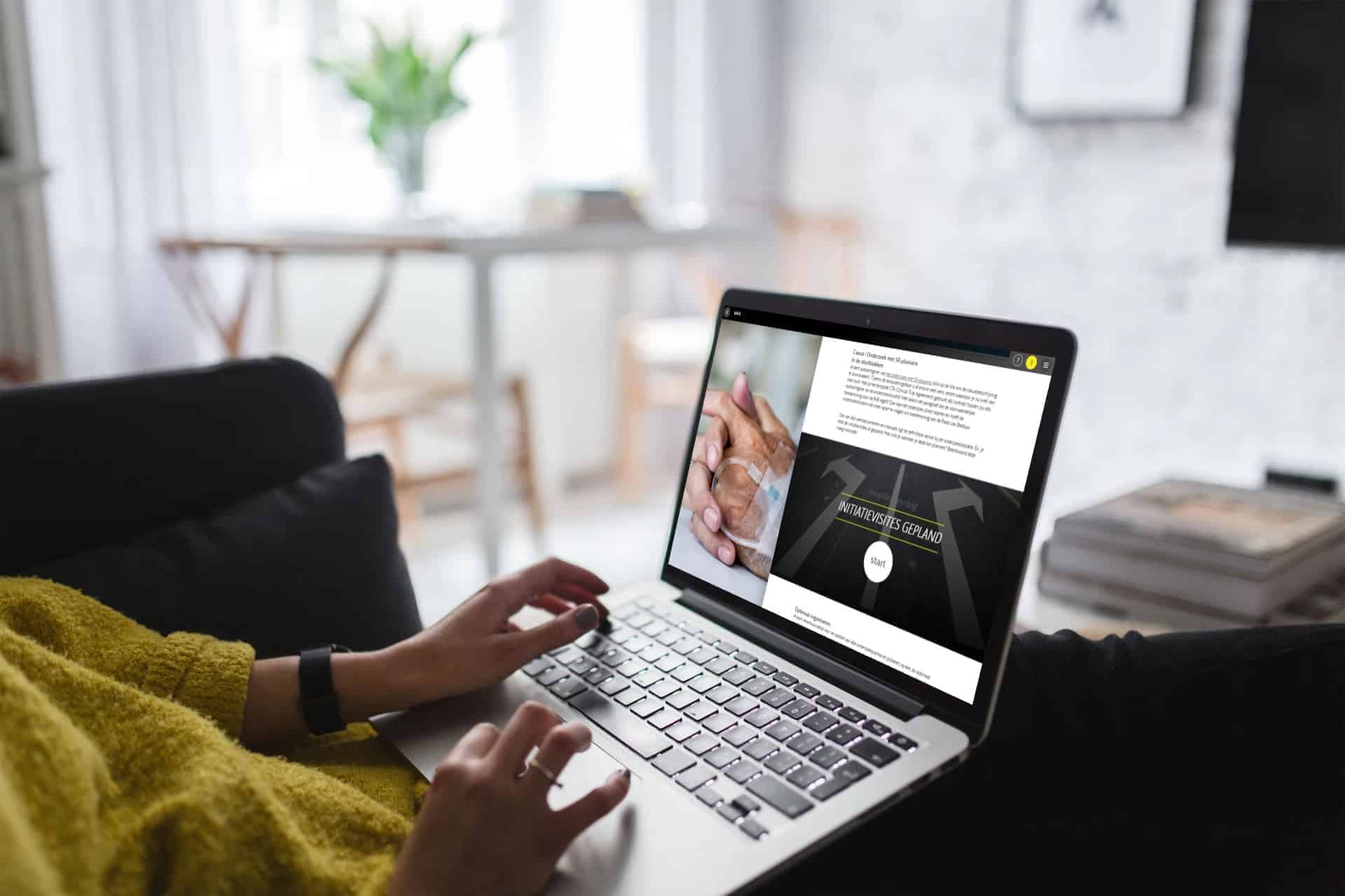
recently launched
-

Training Procedure Lokale haalbaarheid en toepassing Verklaring Geschiktheid Onderzoeksinstelling (VGO) voor Onderzoeksinstellingen & Sponsoren.
€ 50.00 -

Introductory Course to the Site Suitability Procedure for Clinical Trials in the Netherlands
€ 50.00 -

Expert ICH GCP Course for Sites in International Clinical Trials
€ 99.00 -
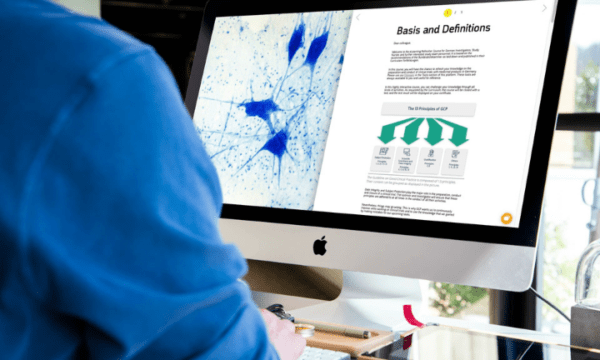
Refresher ICH GCP Course for Clinical Drug Trials in Germany
€ 199.00
ALl courses
Quick Filter
Showing all 49 results
-

Introductory WMO GCP Course to Design Clinical Trials in the Netherlands
€ 65.00 -

Introductory WMO GCP Course to Prepare Clinical Trials in the Netherlands
€ 65.00 -

Introductory WMO GCP Course to Submit Clinical Trials in the Netherlands
€ 65.00 -

Introductory WMO GCP Course to Start Clinical Trials in the Netherlands
€ 65.00 -

Introductory WMO GCP Course to Conduct Clinical Trials in the Netherlands
€ 65.00 -

Introductory WMO GCP Course to Close Clinical Trials in the Netherlands
€ 65.00 -

Fundamental WMO GCP Course to Start and Conduct Clinical Trials in the Netherlands
€ 99.00 -

Re-Certification WMO GCP Course for Clinical Trials in the Netherlands
€ 125.00 -

Expert In-Company Blended Course for Clinical Trials in Europe
€ 375.00 -

Introductory GCP Course to Design Clinical Trials in Europe
€ 65.00 -

Introductory GCP Course to Prepare Clinical Trials in Europe
€ 65.00 -

Introductory GCP Course to Submit Clinical Trials in Europe
€ 65.00 -

Introductory GCP Course to Start Clinical Trials in Europe
€ 65.00 -

Introductory GCP Course to Conduct Clinical Trials in Europe
€ 65.00 -

Introductory GCP Course to Close Clinical Trials in Europe
€ 65.00 -

Fundamental GCP Course to Start and Conduct Clinical Trials in Europe
€ 99.00 -

Expert Online ICH GCP Course for International Clinical Trials
€ 175.00 -

Introductory ICH GCP Course to Design International Clinical Trials
€ 65.00 -

Introductory ICH GCP Course to Prepare International Clinical Trials
€ 65.00 -

Introductory ICH GCP Course to Submit International Clinical Trials
€ 65.00 -

Introductory ICH GCP Course to Start International Clinical Trials
€ 65.00 -

Introductory ICH GCP Course to Conduct International Clinical Trials
€ 65.00 -

Introductory ICH GCP Course to Close International Clinical Trials
€ 65.00 -

Fundamental ICH GCP Course to Start and Conduct International Clinical Trials
€ 99.00 -

Expert In-Company Blended WMO GCP Course for Clinical Trials in the Netherlands
€ 375.00 -

Expert In-Company Blended ICH GCP Course for International Clinical Trials
€ 375.00 -

Introductory ECTR Course for Clinical Drug Trials in the Netherlands
€ 50.00 -

Expert ECTR Course for Clinical Drug Trials in the Netherlands
€ 95.00 -

Fundamental ECTR Course to Conduct Clinical Drug Trials in Europe
€ 65.00 -

Fundamental ECTR Course to Conduct Clinical Drug Trials in the Netherlands
€ 65.00 -

Fundamental ECTR Course to Review Clinical Drug Trials in Europe
€ 65.00 -

Fundamental ECTR Course to Review Clinical Drug Trials in the Netherlands
€ 65.00 -

Fundamental ECTR Course to Submit Clinical Drug Trials in Europe
€ 80.00 -

Fundamental ECTR Course to Submit Clinical Drug Trials in the Netherlands
€ 80.00 -

Basic WMO GCP Test for Clinical Trials in the Netherlands
€ 25.00 -

Basic ICH GCP Test for International Clinical Trials
€ 25.00 -

Expert WMO GCP Course for Clinical Trials in the Netherlands
€ 175.00 -

Introductory ECTR Course for Clinical Drug Trials in Europe
€ 50.00 -

Expert Online GCP Course for Clinical Trials in Europe
€ 175.00 -

Basic WMO GCP Training for the Pharmacy
€ 549.00 -

Expert WMO GCP Training for the Pharmacy
€ 299.00 -

Expert ECTR Course for Clinical Drug Trials in Europe
€ 95.00 -

Basic CFR Course for Clinical Trials in the USA
€ 65.00 -

Expert ICH GCP Course for Sponsors in International Clinical Trials
€ 99.00 -

Introductory GMP Quality Oversight Course for European Drug Products
€ 99.00 -

Refresher ICH GCP Course for Clinical Drug Trials in Germany
€ 199.00 -

Expert ICH GCP Course for Sites in International Clinical Trials
€ 99.00 -

Introductory Course to the Site Suitability Procedure for Clinical Trials in the Netherlands
€ 50.00 -

Training Procedure Lokale haalbaarheid en toepassing Verklaring Geschiktheid Onderzoeksinstelling (VGO) voor Onderzoeksinstellingen & Sponsoren.
€ 50.00
 NEWSLETTERStay up-to date with the latest news and myGCP updates right here
NEWSLETTERStay up-to date with the latest news and myGCP updates right here
 NEWSLETTERStay up-to date with the latest news and myGCP updates right here
NEWSLETTERStay up-to date with the latest news and myGCP updates right here

